Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sato, Takumi; Otobe, Haruyoshi; Morishita, Kazuki; Marufuji, Takato; Ishikawa, Takashi; Fujishima, Tadatsune; Nakano, Tomoyuki
JAEA-Technology 2023-016, 41 Pages, 2023/09
This report summarizes the results of the stabilization treatments of post-experiment nuclear materials in Plutonium Fuel Research Facility (PFRF) from August 2018 to March 2021. Based on the management standards for nuclear materials enacted after the contamination accident that occurred at PFRF on June 6, 2017, the post-experiment nuclear materials containing plutonium (Pu): samples mixed with organic substances that cause an increase in internal pressure due to radiolysis (including X-ray diffraction samples mixed with epoxy resin and plutonium powder which caused contamination accidents), carbides and nitrides samples which is reactive in air, and chloride samples which may cause corrosion of storage containers, were selected as targets of the stabilization. The samples containing organic materials, carbides and nitrides were heated in an air flow at 650  C and 950
C and 950  C for 2 hours respectively to remove organic materials and convert uranium (U) and Pu into oxides. U and Pu chlorides in LiCl-KCl eutectic melt were reduced and extracted into liquid Cd metal by a reaction with lithium (Li) -cadmium (Cd) alloy and converted to U-Pu-Cd alloy at 500
C for 2 hours respectively to remove organic materials and convert uranium (U) and Pu into oxides. U and Pu chlorides in LiCl-KCl eutectic melt were reduced and extracted into liquid Cd metal by a reaction with lithium (Li) -cadmium (Cd) alloy and converted to U-Pu-Cd alloy at 500  C or higher. All of the samples were stabilized and stored at PFRF. We hope that the contents of this report will be utilized to consider methods for stabilizing post experiment nuclear materials at other nuclear fuel material usage facilities.
C or higher. All of the samples were stabilized and stored at PFRF. We hope that the contents of this report will be utilized to consider methods for stabilizing post experiment nuclear materials at other nuclear fuel material usage facilities.
 (M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd
(M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd using cadmium chloride
using cadmium chlorideHayashi, Hirokazu; Shibata, Hiroki; Sato, Takumi; Otobe, Haruyoshi
Journal of Radioanalytical and Nuclear Chemistry, 332(2), p.503 - 510, 2023/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)The formation of MPd (M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu
(M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu -type GdPd
-type GdPd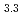 (
( = 0.4081
= 0.4081  0.0001 nm) and NpPd
0.0001 nm) and NpPd (
( = 0.4081
= 0.4081  0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi
0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi -type NpPd
-type NpPd . Chlorination of the MPd
. Chlorination of the MPd (M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl
(M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl formation.
formation.
Soma, Yasutaka; Kato, Chiaki
Dai-68-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.205 - 206, 2021/10
This study investigates the effect of temperature on dissipation behavior of Cl ion within the crevice of stainless steel. Concentration of Cl ion was evaluated by conductivity measured by using sensors installed at crevice specimen. At 50 and 80  C, Cl ions within the crevice of PEEK and Pt dissipated in accordance with concentration diffusion. On the contrary, dissipation speed of Cl ions inside the Type-304L stainless steel were much lower than those anticipated by simple concentration diffusion. This behavior attribute to the anodic dissolution of stainless steel inside the crevice, therefore, to quantitatively understand the effect of temperature on the dissipation behavior, it is necessary to know the anodic dissolution rate and occurrence of localized corrosion. Numerical analysis taking the effect of concentration diffusion and migration into account is also needed.
C, Cl ions within the crevice of PEEK and Pt dissipated in accordance with concentration diffusion. On the contrary, dissipation speed of Cl ions inside the Type-304L stainless steel were much lower than those anticipated by simple concentration diffusion. This behavior attribute to the anodic dissolution of stainless steel inside the crevice, therefore, to quantitatively understand the effect of temperature on the dissipation behavior, it is necessary to know the anodic dissolution rate and occurrence of localized corrosion. Numerical analysis taking the effect of concentration diffusion and migration into account is also needed.
Hata, Kuniki; Inoue, Hiroyuki*
Journal of Nuclear Science and Technology, 56(9-10), p.842 - 850, 2019/09
Times Cited Count:1 Percentile:11.15(Nuclear Science & Technology)To investigate the effect of dissolved species from steels on the radiolysis processes of Cl , radiolysis simulations of solutions containing both Cl
, radiolysis simulations of solutions containing both Cl and Fe
and Fe were carried out. The results showed that the generation of radiolytic products (H
were carried out. The results showed that the generation of radiolytic products (H O
O , O
, O and H
and H ) increased mainly by the addition of Fe
) increased mainly by the addition of Fe , and a drop in the pH was caused by the hydrolysis of Fe
, and a drop in the pH was caused by the hydrolysis of Fe . This pH drop enhanced the reactivity of Cl
. This pH drop enhanced the reactivity of Cl with
with  OH, which induced additional generation of H
OH, which induced additional generation of H O
O and O
and O . These results show that low concentrations of Cl
. These results show that low concentrations of Cl (1
(1  10
10 mol/dm
mol/dm = 35ppm) in the presence of Fe
= 35ppm) in the presence of Fe could influence the generation of H
could influence the generation of H O
O and O
and O during water radiolysis. However, it is considered that these effects of Fe
during water radiolysis. However, it is considered that these effects of Fe and low concentration of Cl
and low concentration of Cl on water radiolysis are less important for corrosion of steels due to the low concentrations of H
on water radiolysis are less important for corrosion of steels due to the low concentrations of H O
O and O
and O generated. The other process, such as dissolution of iron enhanced by FeOOH, might predominantly induce corrosion under the conditions of solutions with low concentrations of H
generated. The other process, such as dissolution of iron enhanced by FeOOH, might predominantly induce corrosion under the conditions of solutions with low concentrations of H O
O and O
and O .
.
Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Ito, Yuta; Shirai, Kaori*; Suzuki, Hayato; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; et al.
Journal of Radioanalytical and Nuclear Chemistry, 320(3), p.633 - 642, 2019/06
Times Cited Count:1 Percentile:11.15(Chemistry, Analytical)An isothermal gas-chromatographic (IGC) device has been developed and tested for on-line gas phase studies of volatile oxychlorides of short-lived group-5 transition metals. Radioisotopes of niobium and tantalum, produced in nuclear fusion evaporation reactions, are directly flushed into the IGC setup by an inert gas-jet. Oxychloride compounds are formed by the addition of SOCl and O
and O . Parameters influencing the formation and transport of NbOCl
. Parameters influencing the formation and transport of NbOCl and TaOCl
and TaOCl are investigated. For nuclides with half-lives (
are investigated. For nuclides with half-lives (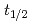 ) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of
) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of  Db(
Db(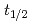 = 34s).
= 34s).
Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Suzuki, Hayato*; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; Nagame, Yuichiro
Inorganica Chimica Acta, 486, p.361 - 366, 2019/02
Times Cited Count:3 Percentile:18.6(Chemistry, Inorganic & Nuclear)The formation of NbOCl and TaOCl
and TaOCl and their adsorption behavior on quartz surfaces was explored by applying an isothermal gas-chromatographic method. Trace amounts of short-lived Nb and Ta isotopes were used. Adsorption enthalpy values (
and their adsorption behavior on quartz surfaces was explored by applying an isothermal gas-chromatographic method. Trace amounts of short-lived Nb and Ta isotopes were used. Adsorption enthalpy values ( ) at zero surface coverage of -
) at zero surface coverage of - (NbOCl
(NbOCl ) = 102
) = 102  4 kJ/mol and -
4 kJ/mol and - (TaOCl
(TaOCl ) = 128
) = 128  5 kJ/mol were determined by analyzing the chromatographic behavior of the Nb andTa complexes with a Monte-Carlo simulation method based on an adsorption-desorption kinetic model.By applying an empirical correlation, the experimental
5 kJ/mol were determined by analyzing the chromatographic behavior of the Nb andTa complexes with a Monte-Carlo simulation method based on an adsorption-desorption kinetic model.By applying an empirical correlation, the experimental  values were successively related to the macroscopic standard sublimation enthalpy,
values were successively related to the macroscopic standard sublimation enthalpy, 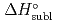 , as a measure of the volatility of each substance. The inferred sublimation enthalpies are in agreement with tabulated thermochemical values. Thus, the linear empirical correlation between
, as a measure of the volatility of each substance. The inferred sublimation enthalpies are in agreement with tabulated thermochemical values. Thus, the linear empirical correlation between  and
and 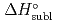 for metal-oxychlorides was updated with the inclusion of the present data. According to the predicted
for metal-oxychlorides was updated with the inclusion of the present data. According to the predicted 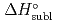 (DbOCl
(DbOCl ), a
), a  (DbOCl
(DbOCl ) value of 135
) value of 135  2 kJ/mol was extrapolated. The future accomplishment of comparative studies with DbOCl
2 kJ/mol was extrapolated. The future accomplishment of comparative studies with DbOCl under the same experimental conditions will provide valuable information on the volatility trend in Group-5 elements, together with an indication on the magnitude of relativistic effects on the electronic structure of dubnium.
under the same experimental conditions will provide valuable information on the volatility trend in Group-5 elements, together with an indication on the magnitude of relativistic effects on the electronic structure of dubnium.
Ogino, Masataka*; Owaki, Eiji*; Shirase, Mitsuyasu*; Nakayama, Masashi
Konkurito Kogaku Nenji Rombunshu (DVD-ROM), 39(1), p.703 - 708, 2017/07
no abstracts in English
 -ray irradiation
-ray irradiationMotooka, Takafumi; Ueno, Fumiyoshi
Zairyo To Kankyo, 64(6), p.220 - 223, 2015/06
Corrosion behavior of carbon steel in chloride aqueous solutions under a low dose rate was investigated by corrosion test using chloride aqueous solutions with different chloride concentration at a dose rate of 500 Gy/h. The corrosion rate of carbon steel increased by the irradiation, and the corrosion rate had the maximum value at a certain chloride concentration. The oxidants produced by radiolysis of chloride aqueous solution enhanced the corrosion of carbon steel. The main oxidants were oxygen and hydrogen peroxide, and the diffusion process of oxidants controlled the corrosion of carbon steel under irradiation. There was a positive correlation between the dependence of corrosion rate and chloride concentration and the dependence of oxidant concentration and chloride concentration.
Shirai, Osamu*; Yamana, Hajimu*; Arai, Yasuo
Journal of Alloys and Compounds, 408-412, p.1267 - 1273, 2006/02
Times Cited Count:39 Percentile:84.47(Chemistry, Physical)no abstracts in English
 in LiCl-KCl eutectic melt
in LiCl-KCl eutectic meltOkamoto, Yoshihiro; Yaita, Tsuyoshi; Minato, Kazuo
Journal of Molecular Structure, 749(1-3), p.70 - 73, 2005/07
Times Cited Count:3 Percentile:7.37(Chemistry, Physical)The local structures of molten BiCl and its mixtures in LiCl-KCl eutectic melt were investigated by X-ray absorption fine structure (XAFS) technique. The 1st Bi-Cl correlation in molten pure BiCl
and its mixtures in LiCl-KCl eutectic melt were investigated by X-ray absorption fine structure (XAFS) technique. The 1st Bi-Cl correlation in molten pure BiCl shows covalent nature, since the distance was almost the same as sum of covalent radii of Bi and Cl and the coordination number was almost 3. The similar property was observed also in the mixture of 75% BiCl
shows covalent nature, since the distance was almost the same as sum of covalent radii of Bi and Cl and the coordination number was almost 3. The similar property was observed also in the mixture of 75% BiCl with LiCl-KCl eutectic melt. Drastic change was detected in 25% BiCl
with LiCl-KCl eutectic melt. Drastic change was detected in 25% BiCl mixture melt. The 1st Bi-Cl distance was sum of ionic radii in molten 25% BiCl
mixture melt. The 1st Bi-Cl distance was sum of ionic radii in molten 25% BiCl melt. The results suggest that BiCl
melt. The results suggest that BiCl changes from molecular liquid to ionic by mixing with alkali chlorides.
changes from molecular liquid to ionic by mixing with alkali chlorides.
Nakamura, Akio; Akabori, Mitsuo; Ogawa, Toru; Huntelaar, M. E.*
Physica B; Condensed Matter, 359-361, p.1021 - 1023, 2005/04
Times Cited Count:1 Percentile:6.23(Physics, Condensed Matter)no abstracts in English
 systems
systemsOkamoto, Yoshihiro; Shiwaku, Hideaki; Yaita, Tsuyoshi; Suzuki, Shinichi; Minato, Kazuo; Tanida, Hajime*
Zeitschrift f r Naturforschung, A, 59a(11), p.819 - 824, 2004/11
r Naturforschung, A, 59a(11), p.819 - 824, 2004/11
The local structure of molten CdCl and CdCl
and CdCl -KCl mixture was investigated by high-temperature XAFS technique. The nearest Cd-Cl distance decreases by melting. Similarly, the coordination number decreases from 6 to 4. It suggests that there is (CdCl
-KCl mixture was investigated by high-temperature XAFS technique. The nearest Cd-Cl distance decreases by melting. Similarly, the coordination number decreases from 6 to 4. It suggests that there is (CdCl )
) tetrahedral complex ion in the melt. It is concluded that the local structure is kept also in the mixture melt, since the same XAFS result as the pure melt was obtained.
tetrahedral complex ion in the melt. It is concluded that the local structure is kept also in the mixture melt, since the same XAFS result as the pure melt was obtained.

Okamoto, Yoshihiro; Yaita, Tsuyoshi; Minato, Kazuo
Journal of Non-Crystalline Solids, 333(2), p.182 - 186, 2004/02
Times Cited Count:11 Percentile:68.51(Materials Science, Ceramics)The local structure of solid and molten SrCl was investigated by X-ray absorption fine structure(XAFS) technique. A superionic conduction state was observed below the melting point. The XAFS data at molten state was obtained at 1000
was investigated by X-ray absorption fine structure(XAFS) technique. A superionic conduction state was observed below the melting point. The XAFS data at molten state was obtained at 1000 C. According to the curve fitting analysis, the nearest Sr
C. According to the curve fitting analysis, the nearest Sr -Cl
-Cl distance and the coordination number are 2.99
distance and the coordination number are 2.99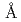 and 6.6 in molten state. XAFS functions calculated from molecular dynamics simulation results were copmpared with the experimental XAFS data.
and 6.6 in molten state. XAFS functions calculated from molecular dynamics simulation results were copmpared with the experimental XAFS data.
Sato, Tadashi; Okamoto, Yoshihiro
Zeitschrift f r Naturforschung, A, 58a(2-3), p.183 - 185, 2003/02
r Naturforschung, A, 58a(2-3), p.183 - 185, 2003/02
Density of molten terbium chloride is first measured by gamma-ray attenuation method as a function of temnperature ranging from 993 to 1213K. Empirical equation for the density of the melt is found to be 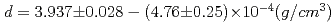 .
.
Shirai, Osamu*; Yamana, Hajimu*; Iwai, Takashi; Arai, Yasuo
Proceedings of Nuclear Fuel Cycle Technologies Closing the Fuel Cycle (CD-ROM), 7 Pages, 2003/00
no abstracts in English
Okamoto, Yoshihiro; Akabori, Mitsuo; Ito, Akinori; Ogawa, Toru
Journal of Nuclear Science and Technology, 39(Suppl.3), p.638 - 641, 2002/11
We report local structural features of molten UCl with LiCl-KCl eutectic probed by the U L
with LiCl-KCl eutectic probed by the U L -edge XAFS(X-ray absorption fine structure). The XAFS measurements were performed in a transmission mode at the BL27B station of the Photon Factory(High Energy Accelerator Organization, Tsukuba, JAPAN). Sample prepared by chlorination of uranium hydride and then reduction with zinc powder was sealed in a quartz cell under reduced pressure. The nearest U
-edge XAFS(X-ray absorption fine structure). The XAFS measurements were performed in a transmission mode at the BL27B station of the Photon Factory(High Energy Accelerator Organization, Tsukuba, JAPAN). Sample prepared by chlorination of uranium hydride and then reduction with zinc powder was sealed in a quartz cell under reduced pressure. The nearest U -Cl
-Cl distance and the coordination number of Cl
distance and the coordination number of Cl around U
around U ion were obtained by a curve fitting of the 1st shell XAFS function k
ion were obtained by a curve fitting of the 1st shell XAFS function k
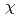 (k). The pair potential in the U
(k). The pair potential in the U -Cl
-Cl correlation was evaluated from XAFS simulation by combinational use of the MD and the FEFF8. In addition, valence state of uranium in the melt was evaluated by XANES(X-ray absorption near edge structure) spectra.
correlation was evaluated from XAFS simulation by combinational use of the MD and the FEFF8. In addition, valence state of uranium in the melt was evaluated by XANES(X-ray absorption near edge structure) spectra.
Okamoto, Yoshihiro; Akabori, Mitsuo; Motohashi, Haruhiko*; Ito, Akinori; Ogawa, Toru
Nuclear Instruments and Methods in Physics Research A, 487(3), p.605 - 611, 2002/07
Times Cited Count:27 Percentile:83.24(Instruments & Instrumentation)Measurement system for the high temperature XAFS was developed for investigating the local structure of hygroscopic molten salts like rare earth halides. Solid sample was enclosed in the upper tank of the quartz cell having a sandglass shape under reduced pressure to avoid oxygen and moisture. The measurement was carried out at the electric furnace of the highest arrival temperature 1273K. After melting, the sample runs down through the melt path with 0.1mm(or 0.2mm) thickness to the lower tank. The minimum energy which could be measured was about 10keV mainly due to the absorption of the quartz. It was confirmed that the measurement of the expensive hygroscopic sample became possible in the present work.
Yaita, Tsuyoshi; Okamoto, Yoshihiro
Hoshako, 14(1), p.42 - 51, 2001/02
no abstracts in English
Haba, Hiromitsu
Kagaku To Kogyo, 54(5), P. 590, 2001/00
no abstracts in English
; ; ; ;
JNC TN9400 2000-066, 52 Pages, 2000/06
Phase I of feasibility studies on commercialized fast reactor system is being peformed for two years from Japanese Fiscal Year 1999. In this report, results of the study on fluid fuel reactors (especialiy a molten salt fast breeder reactor concept) are described from the viewpoint of technical and economical concerns of the plant system design. ln JFY1999, we have started to investigate the fluid fuel reactors as alternative concepts of sodium cooled FBR systems with MOX fuel, and selected the unique concept of a molten chloride fast, breeder reactor, whose U-Pu fuel cycle can be related to both light water reactors and fast breeder reactors on the basis of present technical data and design experiences. We selected a preliminary composition of molten fuel and conceptual plant design through evaluation of technical and economical issues essential for the molten salt reactors and then compared them with reference design concepts of sodium cooled FBR systems under limited information on the molten chloride fast breeder reactors. The following results were obtained. (1)The molten chloride fast breeder reactors have inherent safety features in the core and plant performances, ad the fluid fuel is quite promising for cost reduction of the fuel fabrication and reprocessing. (2)On the other hand, the inventory of the molten chloride fuel becomes high and thermal conductivity of the coolant is inferior compared to those of sodium cooled FBR systems, then, the size of main components such as lHX's becomes larger and the amount of construction materials is seems to be increased. (3)Furthermore economical vessel and piping materials which contact with the molten chloride salts are required to be developed. From the results, it is concluded that further steps to investigate the molten chloride fast breeder reactor concepts are too early to be conducted.